Detecting and preventing Colorectal cancer
This article was originally written and submitted as part of a Canada 150 Project, the Innovation Storybook, to crowdsource stories of Canadian innovation with partners across Canada. The content has since been migrated to Ingenium’s Channel, a digital hub featuring curated content related to science, technology and innovation.
Every week about 423 Canadians are diagnosed colorectal cancer (CRC) and a 175 die from it. In Canada it’s the second most commonly diagnosed and globally it’s the third most common. CRC is highly treatable and 90% preventable if caught early—but nearly 50% of cases aren’t.* Early detection is critical, yet current screening options are either not the most accurate and not patient friendly or invasive and expensive:
- Stool test – 5-15% accurate in detecting colonic polyps (CRC precursor) and often not competed by patients because of format.
- Colonoscopy – invasive, costly and usually involves patient sedation.
Avoiding cancer before it starts
A University of Alberta research discovery led to a diagnostic innovation that dramatically increases detection accuracy, patient compliance and therefore colorectal cancer prevention. The technology is based on the emerging science of metabolomics, the study of small molecules or biomarkers (called metabolites) that can help identify what’s happening inside your body, and help determine your health, nutrition, infection and disease status.
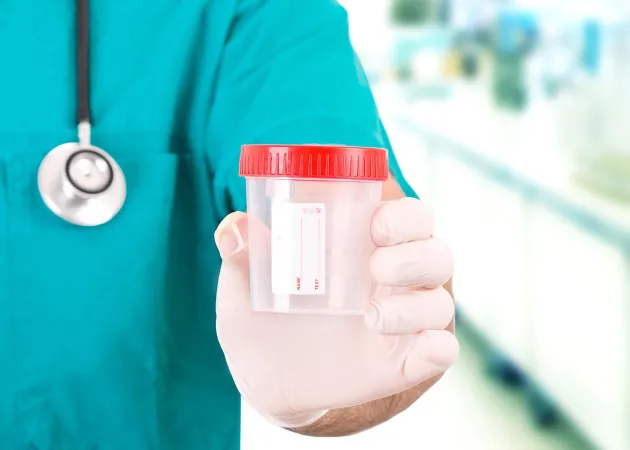
Researchers, Richard Fedorak and Haili Wang, discovered that metabolites could detect colonic polyps (CRC precursors) far more accurately than currently used stool tests, so they developed a urine test to detect these polyps. Their goal—avoiding ‘cancer’ before it starts.
From lab to patients
In 2010, University of Alberta spin-off, Metabolomic Technologies Inc (MTI) was created to move the technology out of the lab and into the hands of doctors and patients. The company developed and launched PolypDx™ a urine test that detects pre-cancerous polyps with over 70% accuracy (vs. stool test).
In fall 2016, Edmonton based MTI signed exclusive licensing deals to distribute the test in 17 U.S. States. They have also completed a clinical trial in China. MTI plans to develop other tests based on this technology, to diagnose other health conditions such as breast cancer, prostate cancer, celiac, IBS, acid reflux, and more.
Transcript
About 475 Canadians are diagnosed with colon cancer (CRC) weekly, and 175 die from it. CRC is a common cancer but is 90% preventable and treatable if caught early. Only about 39% of cases are. A UAlberta research driven innovation will help change that with a urine test that will dramatically increase detection accuracy and patient use. UAlberta spin-off Metabolomic Technologies Inc (MTI) just sealed a US licensing and distribution deal for the test. The company is now exploring the Chinese/European market and planning on new products based on their technology.